Why We Need to Supplement Our Vitamin C Intake
With very few exceptions, all animal species synthesize vitamin C in their bodies. Man does not. The generally accepted theory is that a mutation occurred some tens of millions of years ago to the ancestors of modern man that disabled this synthesis process (see the article C no Vitamin for additional discussion on this). Natural selection dictates that there was adequate C from dietary sources to allow a reasonable level of health, otherwise the mutation could not have taken hold. Indeed, natural selection favors those organisms with only the absolutely necessary machinery. A reader from Arizona State University, Tatiana Covington discussed this in a letter to me thus:
Scurvy is actually a species-wide enzyme deficiency disorder, exactly like Tay-Sachs, gauchers, or Niemann-Pick Type C. There are 4 enzymes which convert glucose into ascorbic acid–but we can only make the first 3. The gene for the 4th–gulonolactone oxidase–is massively damaged and totally useless. Thus we cannot make the last enzyme, and hence cannot turn gulonolactone, which is almost useless against reactive oxygen species, into ascorbic acid, which is the centerpiece of antioxidant, antiradical defense of essentially all air-exposed life on Earth. Without it, indeed, the biosphere would collapse and Earth would be a dead world within a century.
Our antioxidant protection is thus shot to hell. The correct vitamin C level for a human being–a mammal–is c. 2000-3000 mg/kg, and the correct production rate, typical for mammals, is 60 mg-kg/day. But we lack LO, and so our level is c. 25 mg/kg and our synthesis rate is 0. By the way, we are very poor absorbers of C, and thus find it almost impossible to get past 30 mg/kg. (see chart below, Rusty)
Thus the correct path is obvious: reinstall the *gene* for GLO and be done with it, thus curing the universal scurvy. Mammals with the enzyme–which is almost all of them–can live 8-10 times their maturity age. Mammals without it have a hard time reaching 3-4 Ma. Thus this shows that reinstallation of the GLO gene would extend the human life span to c. 300 years.
One mistake, one missing enzyme, one blocked pathway–and you are dead. Ask any hemophiliac.
Our diets have changed dramatically since then. We live in much less hospitable climates. We don’t pick fresh fruits from trees or eat fresh, raw meat, both excellent sources of vitamin C, like our primate ancestors. Our diets are almost universally lacking in nutritional value. Please see the Food Sources Chart. For an excellent discussion of the value of supplementation in general, please visit Healthy.net.
Vitamin C is also not stored well in our bodies. We use it constantly and yet our intake is meager. Only with regular supplementation in quantities that fill our body’s every need will we approach optimum health and the elimination of disease.
Dr. Cathcart talks about how C functions in our bodies depending on the level we are getting (i.e. enough to prevent scurvy, etc.) at the following link – The Third Face of C.
So then the question remains, how much should I take?
Ester-C
There has been an increased popularity in a specific form of vitamin C called “Ester-C.” While it is much more expensive than “ordinary” vitamin C, it does appear to be more bioavailable, which means that your system can utilize a higher percentage of an individual dose. I refer you to a write-up of a study that tends to confirm this, Reference on Ester-C Vitamins.
One of the studies referred to suggests that Ester-C could be three to four times as bioavailable as ascorbic acid. This was at relatively low dosages and I would wonder if this would continue at higher dosages or if a difference in the serum level limit would remain as indicated, I doubt it.
How Much to Take?
“The RDA (U. S. government Recommended Daily Allowance) is the nutritional equivalent of the minimum wage.”
– Dr. Joel Evans
The Center for Women’s Health
The amount of C an individual requires is not determined by the absence of scurvy but by the level that promotes optimum health. Simply put we need to take as much Vitamin C as our systems need to promote optimum health (please read my page C No Vitamin). We have discussed the bowel tolerance limit, the amount you can take without diarrhea-like symptoms. Dr. Cathcart discusses this at Vitamin C Dosage in Disease. Since most animals synthesize vitamin C, how much they produce should provide a clue to how much we need. An excellent article, VITAMIN C: How much is enough? about this is available on the Vitamin C Foundation page. Animals vary widely, and they may not always produce enough for optimum health when under stress. The chart below shows this large variance.
| Daily Production of Ascorbate in Animals | ||
| Animal | Milligrams/Kg Body Weight/per Day | Man’s Equiv. per Day |
| Snake | 10 | 700 |
| Tortoise | 7 | 490 |
| Mouse | 275 | 19,250 |
| Rabbit | 226 | 15,820 |
| Goat | 190 | 13,300 |
| Rat | 150 | 10,500 |
| Dog | 40 | 2800 |
| Cat | 40 | 2800 |
Notice that dogs and cats are low producers (relatively) and that they are more susceptible to vitamin C deficiency related problems. The column headed man’s equivalent shows how much a 150 pound person would produce at the rate of that animal. As you can see it is not totally out of the question that 20,000 mg per day may be required for optimum health, especially when exposed to significant stress.
“The medical profession itself took a very narrow and very wrong view. Lack of ascorbic acid caused scurvy, so if there was no scurvy there was no lack of ascorbic acid. Nothing could be clearer than this. The only trouble was that scurvy is not a first symptom of a lack but a final collapse, a premortal syndrome and there is a very wide gap between scurvy and full health. “
– Albert Szent-Gyorgyi
Nobel-prize winner for his discovery of vitamin C
Everyone is different. Different foods don’t agree with different people. Some people get drunk after two drinks (me). Others can tolerate much more. That’s why drug dosage is a tricky business. These differences are referred to as “biochemical individuality”.
The amount of C that you need will vary according to your body’s need at any particular time. You will become sensitive to how much you can take. I also advise that if you start to feel sick, you start taking one or more 1000 mg tablets every hour (see the Colds page). Again, you will need to judge for yourself. I have just added an account of my own experience trying to balance the right amount of vitamin C to ward off the flu. It isn’t easy. Please check out “A Case of the Flu”.
As a point of reference, I take about 14,000 mg per day in two doses when I’m feeling fine. Also, visit Megadoses of Vitamin C , a paper done to analyze the usefulness and potential danger of taking mega-doses of vitamin C and the editorial Vitamin C, RDA’s and Politics by Steven Wm. Fowkes from the August 1, 1996 issue of Smart Drug News, reprinted at the Cognitive Enhancement Research Institute website.
Generally, I tell people to try to take as much as they can, several times a day. I get a powdered sodium ascorbate. This is non-acidic, has almost no taste, and is easy tolerate. I take it in a little water in the morning and at night. My favorite blogger, Steve Brown, suggests using the governments Upper Limits (UI) recommendations as a starting point. This is an excellent idea. The UIs were established to suggest a level where a supplement would be safe and free of side-effects for most anyone. It is an outstanding thought to use this level as a starting point and increase from there to a level that best works for you to promote optimum health. For vitamin C the UI is 2000 mg per day.
I very often hear people say that any excess C that one ingests is at best wasted or at worst dangerous. First, let me assure you that vitamin C is completely nontoxic. Except for some gastrointestinal distress, NO amount has ever been shown to be toxic (see the discussion of side effects below). So what about the stories that taking over 250 mg (or some other relatively low level) is a waste? Tom Matthews of The LIFE EXTENSION FOUNDATION recently explained it this way (edited for clarity, Rusty):
[T]aking large doses of vitamin C is a bit like attempting to fill a pail to the brim where there are no holes in the sides at all below a certain level, but the density of holes grows as we get closer to the top. If you keep pouring it in fast enough, you will still be able to make the level rise. From this viewpoint, it looks very much like you are simply wasting a lot of water which flows out of the holes in the pail to no purpose. However, to carry the analogy a bit further, suppose that the ability of vitamin C to fight a disease, prevent free radical damage, etc. is analogous to the water pressure at the bottom of the pail. When some injury or disease strikes, it may be analogous to the pail springing a leak below the healthy level of holes – even right at the bottom, and again the pressure of the water may be analogous to the power of vitamin C to fight the injury or disease (and if such is the case it may be important to pour the water in even faster – take higher daily doses of vitamin C – under those circumstances). After the disease or injury is fixed/cured (low hole in the pail plugged), then it is reasonable to reduce the inflow back to maintaining a high level ready to fight off another attack.
So, the best advice is to take as much as you can short of any discomfort. This would be like keeping the pail as full as is practical. When a stress is added (pail springs a leak) much more C will be needed, so much more can be taken before discomfort starts. Under severe circumstances, a knowledgeable physician will administer vitamin C intravenously. This may be especially helpful for cancer patients. Dr. Cathcart explains his Vitamin C for IV Use protocol.
Side-effects and considerations
The other side of this question is can you take too much vitamin C? Can vitamin C become harmful? This quality of a substance is called toxicity, the relative degree of being toxic or poisonous. Many substances are required for life a one level and life-threatening at another. Drugs are quite often very helpful at one level and quite dangerous at a level not much higher. To illustrate, look at the chart below. This chart could be a diagram for iron. Iron is required to carry oxygen. Just the same you can die of iron poisoning if you ingest enough. Vitamin A also gets a bad rap for being dangerous, although the width of the area marked “2” is quite wide. Take ten times the recommended dosage of aspirin and you have quickly gone from “2” to “3”.
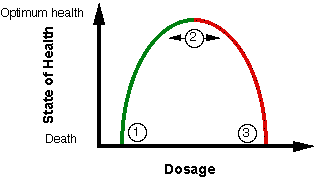
Substances that are only poisonous would have no left side of the diagram, the green area, they would start at “2” and go down from there, sometimes very quickly (e.g. snake venom.) Obviously, these should be avoided! On the other hand, vitamin C doesn’t have a red side to the diagram, it is nontoxic. If you took enough vitamin C to cause diarrhea and continued to take this much, you could die of dehydration, but that’s about as dangerous as C gets. The other dangerous side-effects of vitamin C that are sometimes sited by “authorities” are theories and do not show up in the groups that take large amounts. The potential problems most often brought up as a reason to restrict vitamin C supplementation include kidney stones, excess iron absorption, impaired vitamin B12 status, cellular damage (see the Editorial) or systemic conditioning, sometimes called “rebound scurvy”.
The Safety of Aspirin
To emphasis the point about the relative safety of vitamin C, let’s look more closely at the safety of Aspirin. Most all of us feel Aspirin is “safe”. Certainly not worthy of concern at, or somewhat over, the recommended dosages. We are deluding ourselves about this safety. Quoting from the book The Miracle of MSM by Stanley Jacob, et. al.,
“Data cited at the forum from the “Arthritis, Rheumatism, and Aging Medical Information System” indicate that approximately 76,000 hospitalizations occur each year in the U.S. from gastrointestinal complications produced by non steroidal anti-inflammatory drugs (NSAIDs). An estimated 41,000 hospital admissions and 3,300 deaths involving elderly patients are attributed annually to NSAIDs.”
Aspirin and some of the other over-the-counter pain relievers are NSAIDS. Thirty-three hundred deaths a year! Seventy-six thousand hospital admissions! This is serious, but no one seems to be alarmed. On the other hand, no one has ever died from vitamin C. No one! Ever! At any dosage. Period.
You may also be interested to read an article NSAID deaths by J.S.Hochman MD that discusses deaths from NSAIDs as being equal to or greater than deaths from AIDS!!
Side Effects of Vitamin C
Jerry Rivers of Cornell University (now with the Graduate Division of Nutrition, University of Texas) presented a paper to the Third Conference on Vitamin C entitled “Safety of High-Level Vitamin C Ingestion” where he investigated the potential safety aspects of C mentioned above. I quote the conclusion: “An attempt has been made in this review to select papers that represent opposing views and to present a critical non biased interpretation of the results. This has led to the conclusion that the practice of ingesting large quantities of ascorbic acid will not result in calcium-oxalate stones, increased uric acid excretion, impaired B12 status, iron overload, systemic conditioning, or increased mutagenic activity in healthy individuals.” Dr. Klenner discusses his experience with the dangers of massive amounts of vitamin C in his paper. When Klenner says massive, he means it! He gave individuals intravenous vitamin C treatments of one hundred grams or more per day. Cathcart discusses the relationship between high-dose C and kidney stones in his general article on Vitamin C here.
A study examining the link between vitamin C intake and kidney stone formation published in J Urol, 1996 Jun, 155:6, 1847-51 states:
by Curhan GC, Willett WC, Rimm EB, Stampfer MJ
Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts 02115, USA.
Published – The Journal of Urology, 1996 Jun;155(6):1847-51
PURPOSE: The association between the intake of vitamins C and B6, and kidney stone formation was examined.
MATERIALS AND METHODS: We conducted a prospective study of the relationship between the intake of vitamins C and B6 and the risk of symptomatic kidney stones in a cohort of 45,251 men 40 to 75 years old with no history of kidney calculi. Vitamin intake from foods and supplements was assessed using a semiquantitative food frequency questionnaire completed in 1986.
RESULTS: During 6 years of follow up 751 incident cases of kidney stones were documented. Neither vitamin C nor vitamin B6 intake was significantly associated with the risk of stone formation. For vitamin C the age-adjusted relative risk for men consuming 1,500 mg. daily or more compared to less than 250 mg. daily was 0.78 (95% confidence interval 0.54 to 1.11). For vitamin B6 the age-adjusted relative risk for men consuming 40 mg. daily or more compared to less than 3 mg. daily was 0.91 (95% confidence interval 0.64 to 1.31). After adjusting for other potential stone risk factors the relative risks did not change significantly.
CONCLUSIONS: These data do not support an association between a high daily intake of vitamin C or vitamin B6 and the risk of stone formation, even when consumed in large doses.
A journal article published on Mediconsult, referring to this study states:
Indeed, about one man in 20 was found to consume over 1,500 mg average per day, and it was in this highest consumption group that a reduced risk of kidney stones (22% less than among those who consumed 250 mg or less of Vitamin C) was detected. Apart from this risk reduction at one extreme of the spectrum, no other effect of Vitamin C on stone formation could be construed. (emphasis mine, Ed.)
It would appear that the theory of increased risk of kidney stones from high-dose vitamin C is not substantiated in the real world. It seems from the studies done that high-dose Vitamin C may actually help prevent kidney stones! This subject of kidney stones and vitamin C is a little more complicated than a simple yes/no, even though the above evidence is pretty convincing. Pauling discusses this in his book How to Live Longer and Feel Better. I quote from that:
“It is well known that there are two classes of kidney stones, and that a tendency to form them should be controlled in two quite different ways. The stones of one class, comprising nearly one half of all urinary calculi, are composed of calcium phosphate, magnesium ammonium phosphate, calcium carbonate, or mixtures of these substances. They tend to form in alkaline urine, and persons with a tendency to form them are advised to keep their urine acidic. A good way, probably the best way, to acidify the urine is to take 1 g or more of ascorbic acid each day. Ascorbic acid is used by many physicians for this purpose and for preventing infections of the urinary tract, especially infection by organisms that hydrolyze urea to form ammonia and in this way alkalize the urine and promote the formation of kidney stones of this class.
The kidney stones of the other class, which tend to form in acidic urine, are composed of calcium oxalate, uric acid, or cystine. Persons with a tendency to form these stones are advised to keep their urine alkaline. This can be achieved by taking vitamin C as sodium ascorbate or by taking ascorbic acid with just enough sodium hydrogen carbonate (ordinary baking soda) or other alkalizer to neutralize it.”
IV Vitamin C and Diabetics
I received a letter concerning diabetic patients under IV vitamin C therapy from James A. Jackson, MT(ASCP)CLS, Ph.D., BCLD(AAB) Laboratory Director BioCenter Lab and Senior Research Consultant, Center for the Improvement of Human Functioning. www.brightspot.org www.biocenterlab.org
“Our clinic has been on the forefront of High Dose I.V. Vitamin C for 20 years (Dr. Riordan and The Center staff). A note of caution… any diabetic taking high dose I.V. Vitamin C should be aware that the I.V. vitamin C (ascorbic acid) will be recognized by the electrochemical strips as glucose and give a grossly false positive result…with the ABBOTT instrument “E4..Too high to read with + ketones!” The higher the dose, the higher the reading. The serum hexokinase glucose method is not affected! A diabetic should wait 8 hours after the I.V. before doing a fingerstick. It affects all meters using the electrochemical strip. This finding has been recently published in the JOMM, Dec. 2006 issue. Dr Hunninghake and I are presenting these finding at the Orthomolecular meeting in Toronto, CA in April.
I have several comments to add to this. The most important is that vitamin C is inestimably safer than ANY drug. C does seem to play a role in absorbing iron. Perhaps there is a link between chronic vitamin C deficiency and anemia. It seems to me that the first thing someone with low iron levels should do is increase their vitamin C supplementation. And as far as “systemic conditioning” is concerned, this one really irks me! Chronic vitamin C deficiency leads to a low state of health, no question. To consider that taking supplementation should be avoided because it may make things worse if you stop is ridiculous. You may also wish to read the Message Board Posting that details this issue more. The important thing is to get adequate supplementation. And not to stop! The dangers aren’t from taking C. Indeed, the real dangers are all associated with inadequate dosages!
The most important thing to keep in mind regarding dosages of vitamin C is that the RDA levels of less than 100 mg barely gets you past the area marked 1 in the chart above. While this will prevent scurvy, the vitamin C deficiency disease, and keep you alive in the short term, it won’t get you close to optimum health, the area marked 2 in the chart above.
If you are convinced that vitamin C supplementation is a good idea, there are some things you should be aware of before you start taking 10 grams a day.
Taking too much C at one time will cause diarrhea ( it’s not really diarrhea, but that point will be lost as you run to the bathroom.) This level is refereed to as the bowel tolerance limit. Cathcart talks about this level at Vitamin C Dosage in Disease. Since you’ve been suffering from sub-clinical scurvy since conception, your body is not performing all the enzyme reactions it would if it could. As a result, your bowel tolerance limit will increase over time. I recommend you take 1000 mg tablets once or twice a day to start and increase your dosage every few days, backing off a little if you have the above mentioned symptom.
Also, the stuff the manufacturers add to the C to make it a pill may disagree with you. Try different types if this is the case. Some people are very sensitive to the acidity. Calcium ascorbate tablets are available for you if the ascorbic acid is troubling. Vitamin C powder is what I take because I take a lot (big surprise) and it is very easy to take 8-10 grams at one time this way.
“Today, 290 people in the United States will die from the adverse reaction to prescription medicines.”
– New England Journal of Medicine
1998:279: 1200-1205, 1216-1217
I do not recommend that anyone take lots of the chewable tablets. These are full of sugar and the acidity of the C is hard on the tooth enamel if the exposure level is high. I use the chewables for canker sores, but otherwise I rarely use them.
What about “natural” vitamin C vs. synthesized. Pauling points out that the chemical make-up is identical. If you made a 1000mg tablet entirely from rose hips, the tablet would be as big as a football! Even these tablets are mostly synthesized vitamin C. That is not to say that the natural sources don’t provide nutritional elements not found in the non-natural tablets. The most important thing is the C. Get inexpensive C that agrees with your system and take a lot of it.
